Background
Platinum Group Metals (PGM), including platinum, palladium, and rhodium, are critical components in the catalytic conversion of pollutants produced by internal combustion engines. Historically, palladium has been less expensive than platinum while platinum was the preferred catalytically active metal in aftertreatment systems. Increasing platinum prices led to an increased use of palladium in three-way catalysts and oxidation catalysts. However, demand for palladium has significantly increased as the world’s major economies continue to implement increasingly stringent environmental regulations. Rising cost and increased demand has led researchers to begin developing more efficient methods of utilizing PGM, as well as new catalyst formulations utilizing platinum as the active metal in aftertreatment systems employing PGM.
One method for enhancing the utilization of PGM is the catalyst synthesis technique known as atom trapping. Atom trapping synthesis takes advantage of strong metal-support interactions and high temperature thermal treatments to stabilize highly dispersed, typically atomically dispersed, PGM on a variety of metal oxide supports. Ceria, a metal oxide commonly utilized as the oxygen storage component of three-way catalysts, is well known to have strong interactions with PGM and has demonstrated the capability of stabilizing single atoms of platinum, palladium, and even rhodium at temperatures in excess of 800 °C.
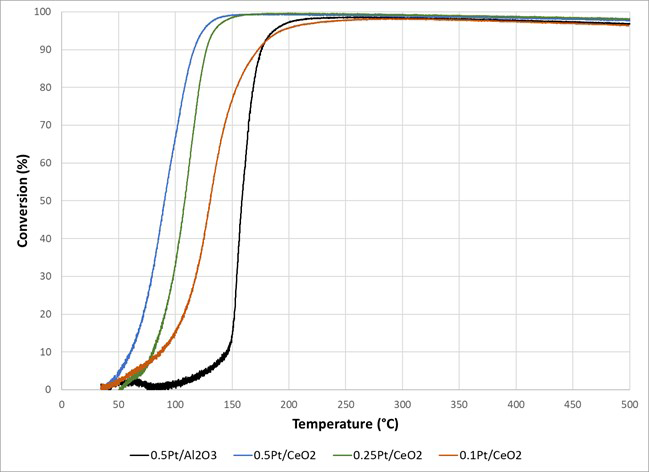
Figure 1: Stainless-steel reactor tube used for powder catalyst testing in USGR system.
Approach
In this work, ceria-supported PGM catalysts were synthesized using the atom trapping technique and compared to a commercially available alumina-supported Pt catalyst. Both Pt and Pd catalysts were prepared using this technique. Catalysts were also prepared using a lower calcination temperature at 550 °C to produce well dispersed Pt clusters without isolated single atoms. Powder catalyst samples were tested in a plug-flow reactor utilizing a modified US Drive protocol for oxidation catalyst testing. Catalysts were tested for light-off behavior of carbon monoxide and ethylene both with and without co-metered water. Sulfur poisoning studies were conducted to elucidate the catalyst response to common diesel exhaust poisons and the ability to easily recover activity through in situ regeneration protocols.
Accomplishments
The ceria-supported catalysts calcined at 550 °C exhibited superior low temperature CO oxidation activity when compared to the control catalyst, two of which converted approximately 100% CO below 150 °C. At comparable loadings of Pt (i.e., 0.5 wt%), the ceria-supported Pt catalysts exhibited a T50 (temperature of 50% conversion) of CO approximately 50 °C lower than the control catalyst with co-fed C2H4 and H2O. The T50 of C2H4 was approximately 25°C lower for ceria-supported catalysts at comparable weight loadings. Catalysts supported on ceria exhibited severe SO2 poisoning with losses of catalytic activity of greater magnitude compared to the control alumina-supported catalyst. A simple regeneration procedure containing CO, C2H4, and H2O was able to recover the activity of Pt supported on ceria without any indication of sintering or significant alteration to the active sites.
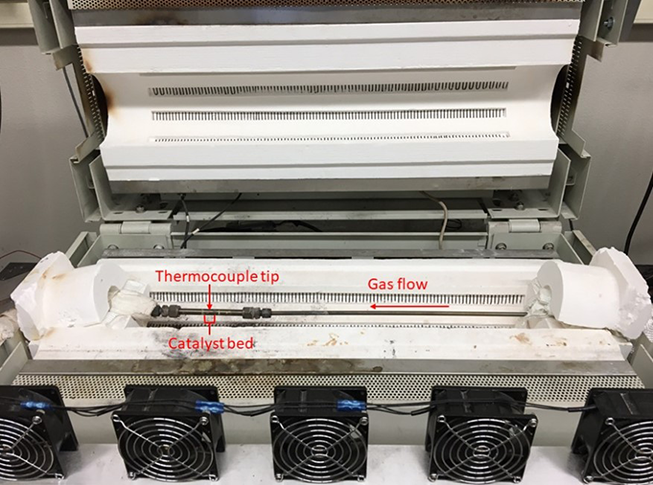
Figure 2: Conversion of 500 PPM CO in 12% O2 and balance N2 with a total flow of 3 L/min.
