Background
Cancer treatment has entered a new era of innovative solutions, such as CAR T-cell therapy, which involves using a patients’ own immune cells, namely T cells, to achieve deep and durable control of cancer. However, current T cell manufacturing is a complex, labor-intensive process requiring a high-cost Class 100 cleanroom facility, which results in about $500,000 costs for a single treatment of personalized CAR T-cell therapy. In addition, current T cell manufacturing uses magnetic microbeads for T-cell purification, activation, and expansion. As the cells are harvested, the beads must be separated from the cells. However, it is difficult to completely remove the beads from the cells so they often are infused into a patient along with T-cells, resulting in a potential safety issue.
Approach
This project, jointly funded by National Institute for Innovation in Manufacturing Biopharmaceuticals or NIIMBL, is to develop an alternative beads-free bioreactor platform that addresses the high cost of current CAR T cell manufacturing and provides a scalable, easy-to-use, and cost-effective technology for using CAR T cell therapy in cancer immunotherapy. The objective of this application is to demonstrate the feasibility and potential advantages of a novel perfusion-based, beads-free bioreactor system through an integrated process/platform development for T-cell selection, activation, and gene transduction.
Accomplishments
In this study, SwRI and its collaborator at the University of Pennsylvania, for the first time, demonstrated that a perfusion-based, beads-free, 3D printed single-use bioreactor was able to activate T cells. For the first time we showed that the bioreactor has the potential to serve as a cell selection/purification pre-filter. For the first time we illustrated that the cells in the bioreactor can be transduced by lentiviral vectors. In addition, we demonstrated that the perfusion-based, beads-free bioreactor has the potential to form an automated, closed-loop system for cost-effective T cell manufacturing.
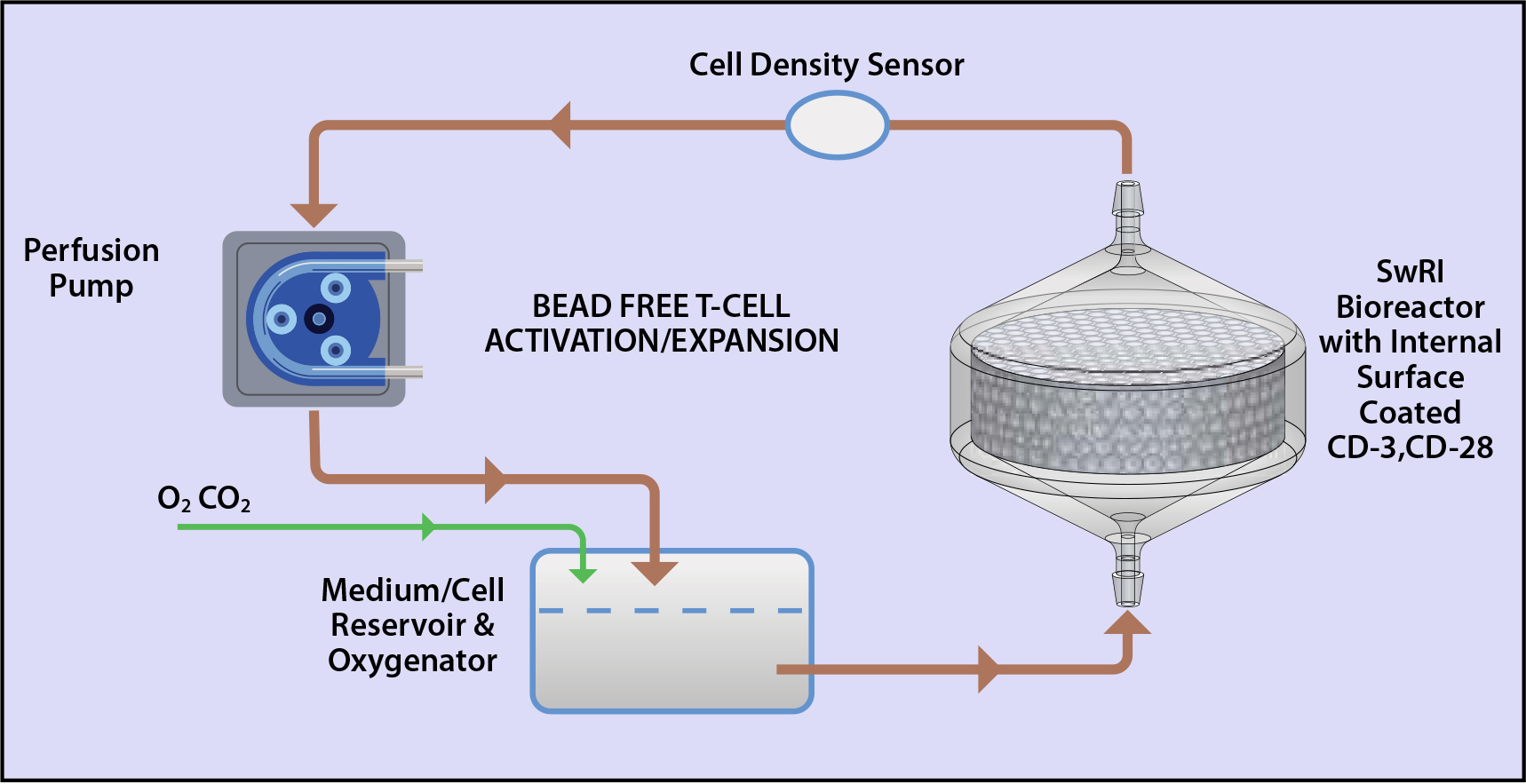
Figure 1: SwRI’s bead-free perfusion-based system offers closed-loop, automated T-cell activation, transduction, and expansion in comparison to the complex, labor-intensive processes typically requiring Class 100 cleanrooms.

