Background
Traumatic brain injury (TBI) is the most common cause of cognitive and behavioral deficits in the U.S and worldwide. Most TBIs are classified as mild-TBI and concussion (C) that are associated with minor symptoms and microscopic damage with no observable pathology on imaging scans, and thus are challenging to be diagnosed. Equally important are asymptomatic sub-concussive (SC) head exposures that when left undiagnosed put the subjects at higher risk of repeated exposures, resulting in cognitive declines and initiation of chronic neurodegenerative disease. C/SC head exposures cause cellular disruptions in the brain which initiate a variety of neuroinflammations and metabolomic dysregulations which have shown to be reflected in variety of biofluids. Recently two blood biomarkers have been approved by FDA for diagnosis of TBI. While minimally invasive blood biomarkers are valuable in diagnosis of concussions, they are less practical in SC exposures in sports and military settings where the rates of the SC are high. Exhaled breath (EB), which expresses various metabolites, proteins, and cytokines, can be a non-invasive alternative approach for these settings. Although EB has recently joined blood and urine as primary biofluids for diagnostic of different diseases like cancer, diabetes and tuberculous; It has not been yet been explored for diagnosis of TBI.
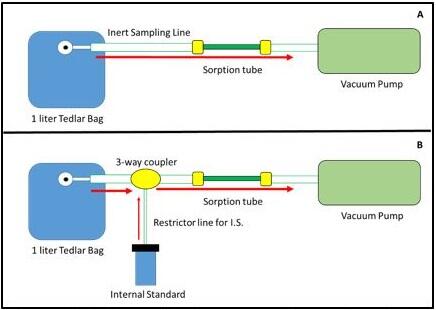
Figure 1. Schematic of gas sampling system (a) and design of gas sampling system with internal standard (b).
Approach
This project explored the use of exhaled breath for the discovery of biomarkers related to TBI. In addition, an in-house gas sampling system (GSS), Figure 1, was developed for the purpose of quantitative and reproducible sampling. Once the GSS was developed and tested, breath samples from animal and TBI patients were collected and analyzed as part of a proof-of-concept study.
Accomplishments
Project R6230 was successful in designing and developing a GSS capable of reproducible and robust sampling metrics. Analytical testing showed relative standard deviations (%RSD) values for a C5-C9 alkane test mixture below 6% for n=20 sample set. The addition of an internal standard allowed for a greater than 3x improvement in %RSD across the test set. The analytical method was optimized and more than 40 samples were analyzed as part of the TBI study. Processed data showed improvements in quantitative calculations as well as linear response. Statistical analysis of collected breath samples proved successful in identifying biomarkers related to TBI in both human and animal models (Figure 2).
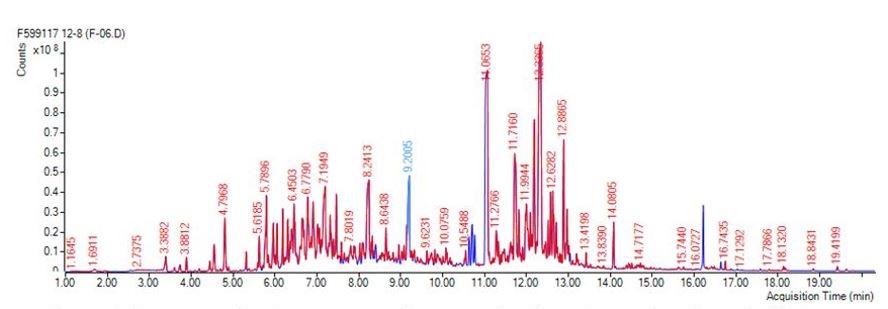
Figure 2. Representative chromatogram from an animal breath sample collected with the gas sampling system and analyzed via gas chromatography and mass spectrometry.
