Background
Our group has been developing novel, state-of-the-art GC-MS instrumentation utilizing MEMS GC columns, fabricated at the University of Michigan (UM), coupled to the MAss Spectrometer for Planetary EXploration (MASPEX). The genesis of micro-GC work at SwRI began with the IR&D program, “Advanced Micro-GC for Space Science Mass Spectrometry” (15-R85918). That support helped us to obtain funding from the Instrument Concepts for Europa Exploration (ICEE) 2 program through NASA Grant #80NSSC19K0611. Over the course of the ICEE 2 project, we have continued to build SwRI’s GC-MS program, and we have also published three papers. Two of these papers provide MEMS GC-MS experimental data for the separation of organics and biological molecules, and a third presents the analytical utility of comprehensive two-dimensional gas chromatography (GC×GC) for space science.
The next step in our technological development is to demonstrate the capability of the MEMS GC columns to separate chiral molecules (enantiomers). Chiral molecules possess the same chemical formula, but its molecular structure is not superimposable. This means that its mirror image is distinguishable from itself (i.e. hand, where the word chirality is derived from the Greek word meaning hand). Selective synthesis of chiral molecules is a hallmark of life, and the preferential occurrence (enantiomeric enrichment) of one chiral form over another is considered strong evidence for the presence of life. Thus, chiral analysis is a critical tool in the search for life in the solar system. Chiral molecules are completely blind to even the highest mass resolution mass spectrometers because they have the exact same molecular weight, but can be separated by gas chromatography (GC) with special chiral stationary phases. Chiral separations by commercial columns with GC and GC-MS is a routine analytical technique and provides us a good reference point to compare our results. We have begun a collaboration with Restek Corporation, who will provide their industry-leading chiral stationary phases to be coated on the MEMS GC columns by UM. We will test the separation capability of the chiral MEMS GC columns here at SwRI with a prototype version of MASPEX for mass spectrometric identification and detection of the separated GC components. A direct comparison will be made to the commercial chiral stationary phase GC columns.
Approach
We obtained commercial chiral stationary phase columns from our collaboration with Restek Corporation. The three chiral stationary phases obtained were: Rt-βDEXm (Catalog # 13100), Rt-βDEXsm (Catalog # 13105), and Rt-γDEXsa (Catalog # 13113). All columns had the following dimensions: 30 m length, 0.25 mm ID, and 0.25 μm film thickness. The three different columns were tested for their ability to separate D- and L-amino acid enantiomers of Alanine (Ala), Valine (Val), and Aspartic Acid (Asp). All columns were inserted in an Agilent 6890 commercial GC oven and a mixture of the D- and L-amino acids were derivatized in dimethyl formamide-dimethyl acetal (DMF-DMA) and injected into the commercial injector. The GC conditions for the separation were as follows: injector temperature of 250 °C, He carrier gas flow rate of 1.2 mL min-1, GC oven temperature 70 °C held for 5 minutes, followed by a 3 °C min-1 ramp to 190 °C and held isothermally at 190 °C for the duration of the separation.
The commercial chiral stationary phase providing the best performance was then selected for coating 3 x 10 m length MEMS GC columns to be connected in series and tested with the same D- and L-amino acid enantiomers. Identical GC separation conditions were used for the MEMS GC columns. The performance of the MEMS GC column setup was directly compared to the commercial column in terms of chromatographic resolution between amino acid enantiomers.
Accomplishments
The D- and L-amino acid enantiomer mixtures were separated on each of the three chiral stationary phase (Rt-βDEXm, Rt-βDEXsm, and Rt-γDEXsa) commercial columns. The best results for the D-and L-amino acid enantiomeric separations were obtained with the Rt-βDEXsm column with slightly less resolution provided by the Rt-γDEXsa column. Separation was observed for the D-and L-amino acids of Ala and Asp and only one peak (enantiomers unresolved) was observed for Val. The overlaid results of the enantiomeric separation on the two commercial columns is displayed in Figure 1. The Rt-βDEXm column provided the lowest resolution and only separated D- and L-Ala.
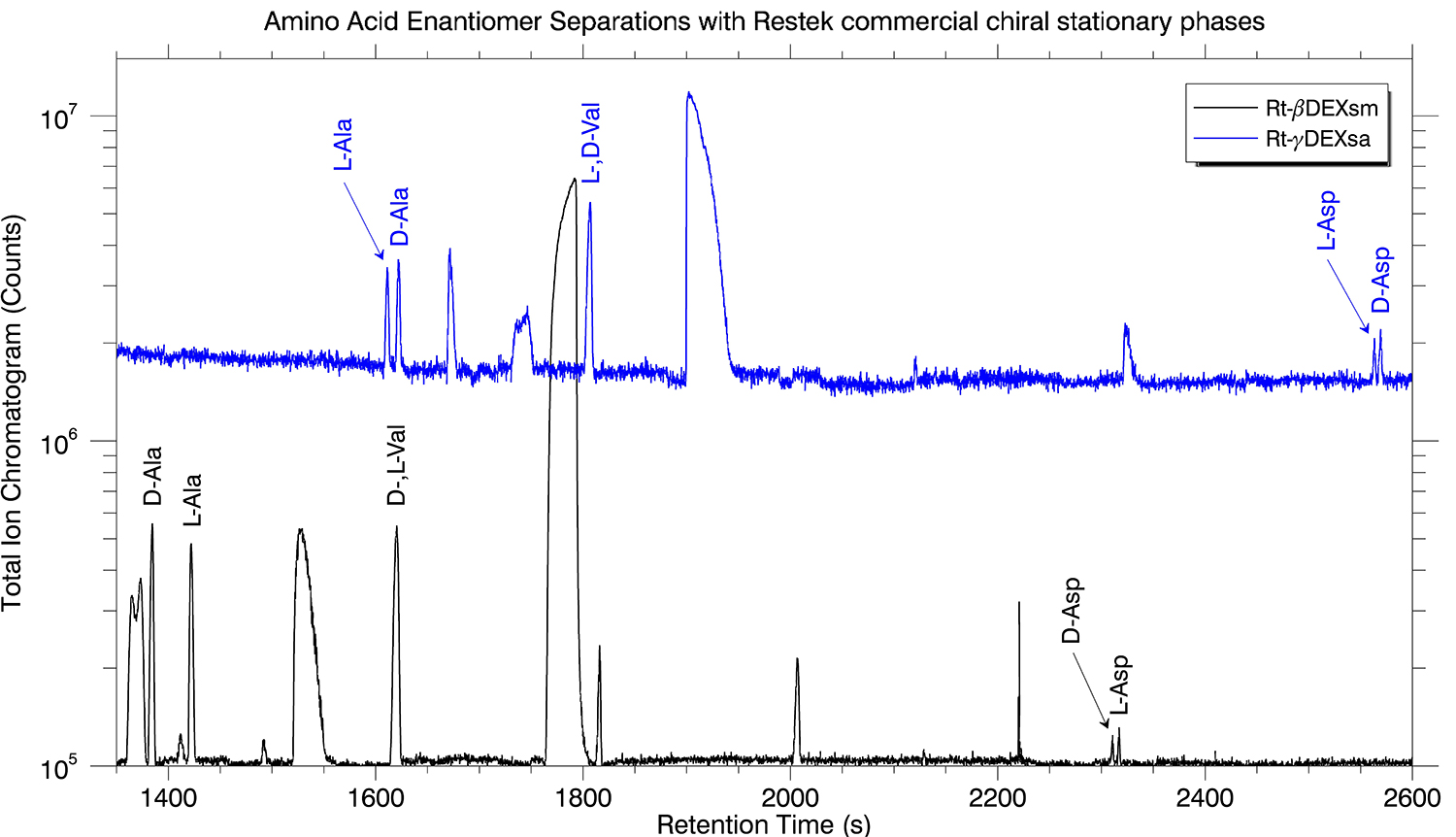
Figure 1: Chromatograms from separation of Ala, Val, and Asp enantiomers (D- and L-) on Restek’s Rt-βDEXsm (black) and Rt-γDEXsa (blue) chiral stationary phase columns.
The Rt-βDEXsm stationary phase was chosen for coating 3 x 10 m length MEMS GC columns at the University of Michigan (UM). Restek provided UM with the recipe to be used for the liquid stationary phase mixture and UM coated this stationary phase on the three MEMS columns using a dynamic coating process. The 3 x 10 m MEMS columns coated with Rt-βDEXsm (Figure 2) were sent to SwRI for testing with amino acid enantiomers and mass spectrometric detection after the chromatographic separation. For the amino acid tests, both the D- and L- enantiomers of individual amino acids were injected and separated on the 3 x 10 m MEMS columns in series.

Figure 2: Photograph of the three MEMS GC columns connected in series and taped down to a stand to be inserted in the GC oven. The inlet to column 1 (R1) is connected to the inlet/injector of the commercial GC and the outlet of column 3 (R3) is connected to a capillary that goes to the ion source of MASPEX through a heated transfer line.
Results of the D- and L-amino acids ran on the 3 x 10 m length MEMS GC columns in series are displayed in Figures 3 and 4. Separation was observed for the D- and L-amino acid enantiomers of Ala and Asp but broad chromatographic peaks with tailing were observed rather than very sharp, Gaussian peaks observed with the commercial columns. It is hypothesized that this is from non-uniform chiral stationary phase coating. Retention time shifts were also observed to faster retention times (to the left) for subsequent injections of all of the amino acid enantiomers. This seems to indicate stationary phase bleed, or instability of the stationary phase. Lastly, the retention times on the MEMS GC columns are much faster (analytes significantly less retained), indicating non-uniform or incomplete stationary phase coating. The performance of the MEMS column was directly compared to the commercial column in terms of provided resolution: D, L-Ala ~1.25 on MEMS column versus 4.65 on commercial column, and D, L-Asp ~0.78 on MEMS column versus 0.976 on commercial column (the larger the number the greater the resolution). In terms of percentage of resolution provided by the MEMS column compared to the commercial column the results were: D, L-Ala performance was ~25-27% versus commercial column, D, L-Asp performance was ~80% versus commercial column. It is clear that improvements to the stationary phase coating process of the MEMS GC columns are required and further investigation of this topic is planned in the future.
The data gathered during this project was used to strengthen a MatISSE proposal submitted to NASA on July 14, 2022 to raise the TRL of the entire MEMS GC-MS system from 4 to 6. The MatISSE proposal opportunity defined the short-term business case for this Targeted IR.
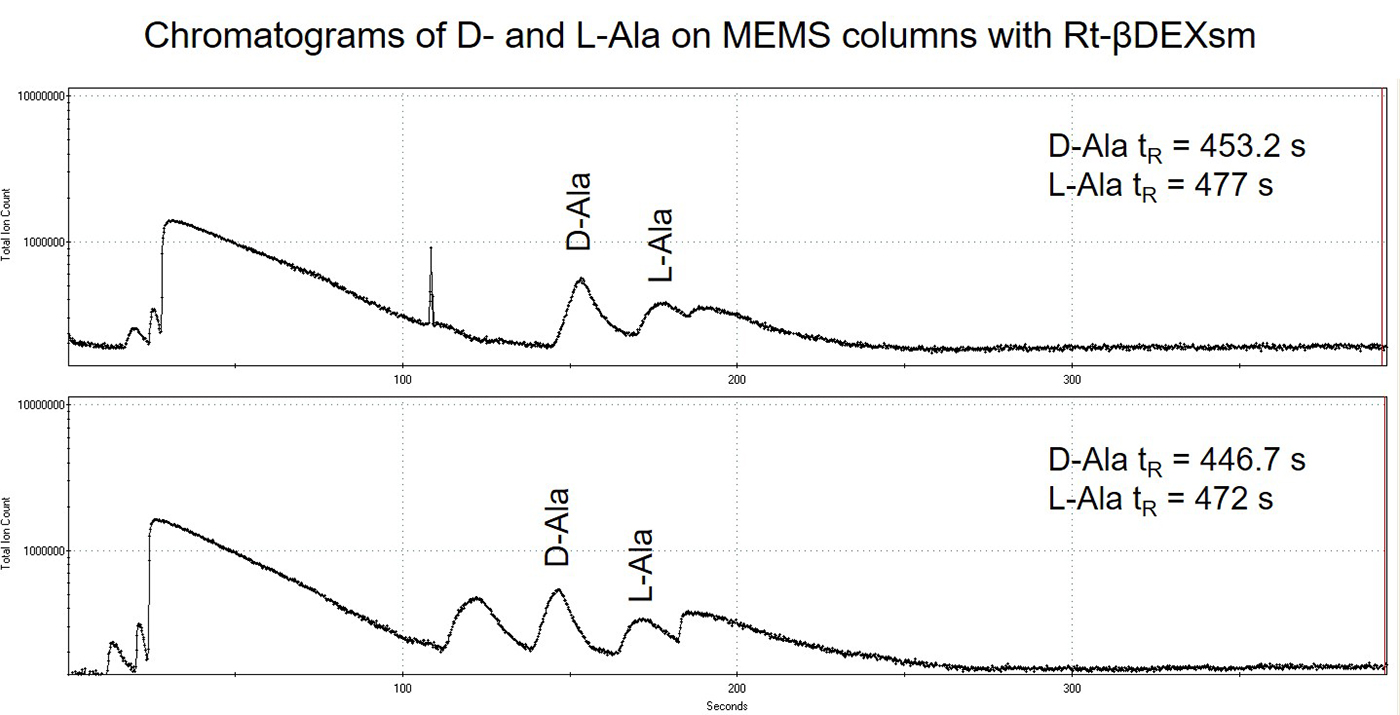
Figure 3: Chromatograms of two subsequent runs of D- and L-Ala on the 3 x 10 m MEMS columns in series coated with Rt-βDEXsm. A delay time of 300 s was used prior to turning on the TOF-MS data acquisition.
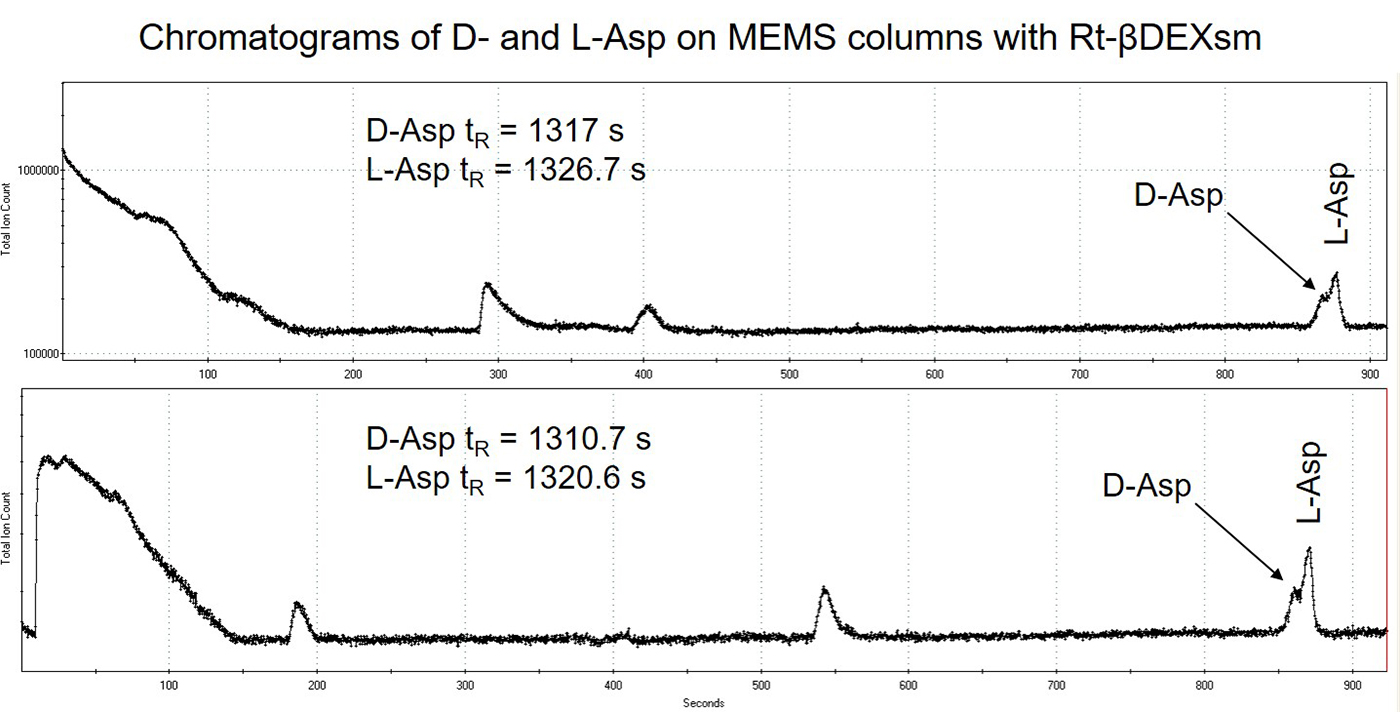
Figure 4: Chromatograms of two subsequent runs of D- and L-Asp on the 3 x 10 m MEMS columns in series coated with Rt-βDEXsm. A delay time of 450 s was used prior to turning on the TOF-MS data acquisition.

