BACKGROUND
Corrosion of carbon steel in the presence of H2S is an important topic in the oil and gas industry that has been studied for more than 50 years. Removing this compound results in not only an increase in the corrosion resistance of carbon steel, but also an improvement of environmental, health, and safety compliance, thereby increasing production efficiency. Triazines are commonly used as H2S scavengers. However, failures in the form of stress corrosion cracking (SCC) have recently been reported when using certain triazines, making it necessary to understand the failure mechanism to mitigate and control this detrimental phenomenon. It has been suggested that SCC is governed by corrosion processes and specifically by the transition from a passive (more protective) surface to an active (less protective) surface. This transition is a complicated function of acid gas concentration (CO2 and H2S) and amine adsorption, which are likely different for different systems. Therefore, characterization of the effect of the triazine/H2S reaction processes on the surface properties of steel is an important factor that needs to be explored.
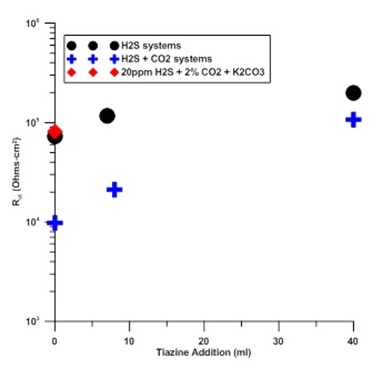
Figure 1: This electrochemical impedance spectroscopy (EIS) graph shows that for all levels of triazine, solutions containing 2 percent CO2 had a higher corrosion rate than solutions without CO2. Note that, in this image, the corrosion rate is the inverse of the charge transfer resistance (Rct).
APPROACH
In this work, we sought to understand the effect of triazine on the electrochemical response of a carbon steel surface in a mixed gas system. Cyclic Voltammetry (CV) was used to qualitatively assess the nature of the carbon steel surface with and without CO2 in the presence of triazine. Changes in surface film properties were also assessed using electrochemical impedance spectroscopy (EIS) in an attempt to quantify changes in the electronic properties of the surface layer. Insight gained into the mechanisms of surface changes will be used by UTSA to define regions where SCC is likely.
ACCOMPLISHMENTS
Cyclic voltammetry was used to identify specific oxidation events associated with FeS formation. However, extreme care must be used in the interpretation because of apparent double layer charging that confounds a critical interpretation of the data. CV scans indicated that increases in triazine additions resulted in a reduction in the formation kinetics of FeO(OH). For the highest triazine additions, the kinetics were reduced enough to provide evidence of the presence of Fe3O4 which may help explain the reduced corrosion rate observed at the highest triazine levels. EIS showed that for all levels of triazine, solutions containing 2 percent CO2 had a higher corrosion rate than solutions without CO2 (Figure 1). Note that corrosion rate is the inverse of the charge transfer resistance (Rct) shown in Figure 1. Additionally, triazine additions had a much stronger effect on corrosion rate for solutions with CO2 additions. Specifically, additions of triazine decreased the corrosion rate for systems with and without CO2 but the effects were greater in the latter case. While pH plays a strong role in affecting corrosion rates for systems with 20 ppm H2S, other factors contribute for systems where CO2 is added. Plausible mechanisms of triazine effects on corrosion rate may include competitive surface adsorption of triazine or its by-products or surface passivation. The extent of triazine effects are modified in the presence of CO2 where additional molecules are available for competitive surface adsorption or H2S scavenger efficiency is reduced.

